Research at the University of Chicago
Maldonado, N., Hou, C., Wuttig, A.* (2025). Submitted.
Papadopoulos, R.,¹ Masters, B.,¹ Kundu, A.,¹ Maldonado, N., Filatov, A., Liu, Y., Kim, T., Galli, G., Wuttig, A.* (2025). Unlocking Mesoscopic Disorder in Graphitic Carbon with Spectroelectrochemistry. Angewandte Chemie International Edition, e202420680. [link] ¹Equal author contribution.
– highlighted in Angewandte Chemie International Edition, Introducing…

Kim, T.,¹ Kim, Y.,¹ Wuttig, A.* (2024). Interfacial Science for Electrosynthesis. Invited Review. Current Opinion in Electrochemistry, 47, 101569. [link] ¹Equal author contribution.

Kunstelj, Š., Darù, A., Sauza-de la Vega, A., Stroscio, G., Edwards, E., Papadopoulos, R., Gagliardi, L., Wuttig, A.* (2024). Competitive Valerate Binding Enables RuO2-Mediated Butene Electrosynthesis in Water. Journal of the American Chemical Society, 146, 30, 20584−20593. [link]

Jiang, N., Darù, A., Kunstelj, Š., Vitillo, J., Czaikowski, M., Filatov, A., Wuttig, A., Gagliardi, L., Anderson, J.* (2024). Catalytic, Spectroscopic, and Theoretical Studies of Fe4S4-Based Coordination Polymers as Heterogenous Coupled Proton–Electron Transfer Mediators for Electrocatalysis. Journal of the American Chemical Society. 146, 17, 12243–12252. [link]
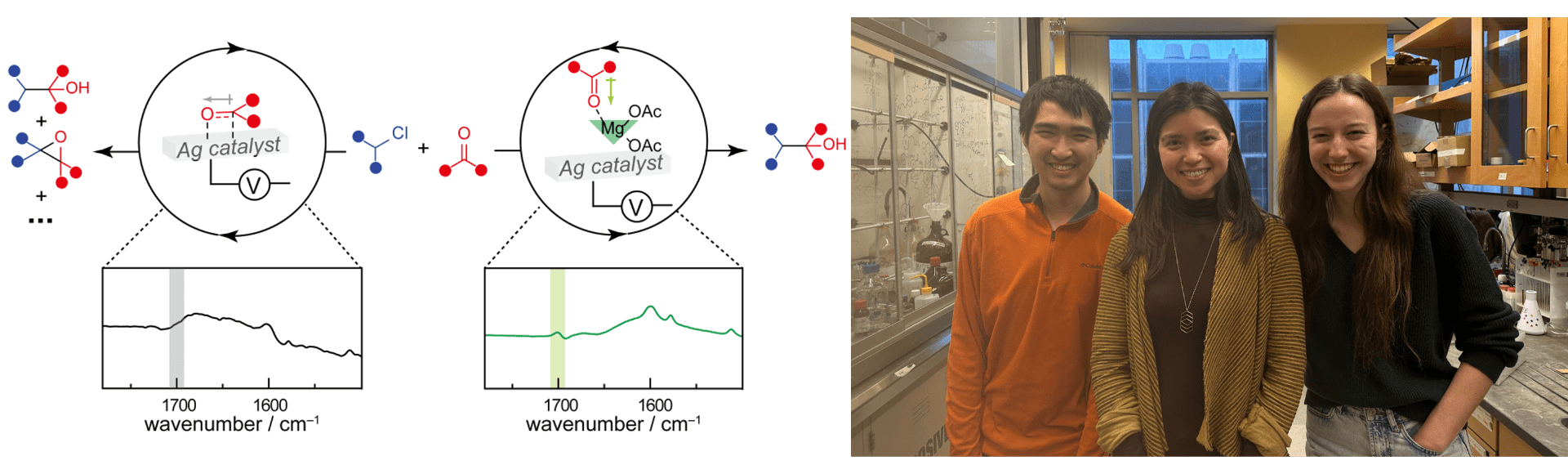
Chen, Q.C., Kress, S., Molinelli, R., Wuttig, A.* (2024). Interfacial Tuning of Electrocatalytic Ag Surfaces for Fragment-Based Electrophile Coupling. Nature Catalysis, 7, 120–131. [link]
– highlighted by University of Chicago News
– highlighted by Nature Catalysis, News & Views
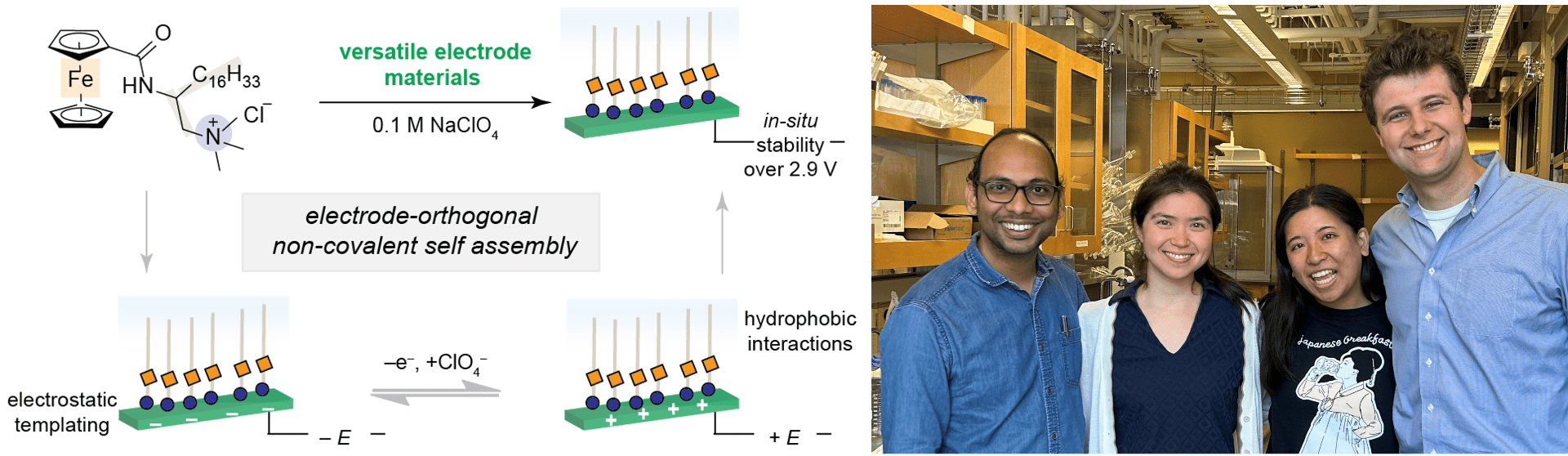
Badgurjar, D.,¹ Huynh, M.,¹ Masters, B., Wuttig, A.* (2023). Non-Covalent Interactions Mimic the Covalent: An Electrode-Orthogonal Self-Assembled Layer. Journal of the American Chemical Society. 145, 32, 17734–17745. [link] ¹Equal author contribution
Wuttig, A.,* Toste, F. D.* (2021). The interface is a tunable dimension in electricity-driven organic synthesis. Natural Sciences, 1 (2), e20210036. [link]
Research Prior to the University of Chicago
Wuttig, A., Derrick, J. S., Loipersberger, M., Snider, A., Head-Gordon, M., Chang, C. J.,* Toste, F. D.* (2021). Controlled Single Electron Transfer via Metal-Ligand Cooperativity Drives Divergent Nickel Electrocatalyzed Radical Pathways. Journal of the American Chemical Society, 143 (18), 6990–7001. [link]
Wuttig, A., Ryu, J. & Surendranath, Y.* (2021). Electrolyte Competition Controls Surface Binding of CO Intermediates to CO2 Reduction Catalysts. The Journal of Physical Chemistry C, 125 (31), 17042–17050. [link]
Ryu, J., Wuttig, A. & Surendranath Y.* (2018). Quantifying Interfacial pH Variation at Molecular Length Scales Using a Concurrent Non-Faradaic Reaction. Angewandte Chemie International Edition, 57 (30), 9300-9304. [link]
Wuttig, A., Yoon, Y., Ryu, J. & Surendranath, Y.* (2017). Bicarbonate is Not a General Acid in Au-Catalyzed CO2 Electroreduction. Journal of the American Chemical Society, 139 (47), 17109-17113. [link]
Wuttig, A., Liu, C., Peng, Q., Yaguchi, M., Hendon, C. H., Motobayashi, K., Shen, Y., Osawa, M. & Surendranath, Y.* (2016). Tracking a Common Surface-Bound Intermediate during CO2-to-Fuels Catalysis. ACS Central Science, 2 (8), 522-528. [link]
Wuttig, A., Yaguchi, M., Motobayashi, K., Osawa, M. & Surendranath, Y.* (2016). Inhibited Proton Transfer Enhances Au-Catalyzed CO2-to-Fuels Selectivity. Proceedings of the National Academy of Sciences, U.S.A., 113 (32), E4585 – E4593. [link]
Wuttig, A., Krizan J., Gu J., Frick, J., Cava. R., & Bocarsly A.* (2016). The Effect of Mg-doping and Cu nonstoichiometry on the Photoresponse of CuFeO2. Journal of Materials Chemistry A, 5 (1), 165-171. [link]
Hall, A., Yoon, Y., Wuttig, A., Surendranath, Y.* (2015). Mesostructure-Induced Selectivity in CO2 Reduction Catalysis. Journal of the American Chemical Society, 137 (47), 14834-14837. [link]
Wuttig, A., Surendranath, Y.* (2015). Impurity Ion Coordination Enhances Carbon Dioxide Reduction. ACS Catalysis, 5(7), 4479-4484. [link]
Gu J., Wuttig, A., Krizan J., Hu, Y., Detweiler Z., Cava R., Bocarsly A.* (2013). Mg-doped CuFeO2 Photocathodes for Photoelectochemical Reduction of Carbon Dioxide. The Journal of Physical Chemistry C, 117 (24), 12415-12422. [link]
Hsia C., Wuttig, A., Yang H.* (2011). An Accessible Approach to Preparing Water-Soluble Mn2+-doped (CdSSe)ZnS (Core) Shell Nanocrystals for Ratiometric Temperature Sensing. ACS Nano, 5 (12), 9511-9522. [link]
